CMS Inspection Report
All Laboratory compliance is the Lab Director’s Responsibility. Theranos was a manufacturer of Technology and a CLIA lab. Neither Holmes or Sunny had any experience in, not any different from Jim Davis CEO of Quest Diagnostics or Adam H. Schechter CEO of LabCorp. They have a whole team for regulatory ie. Lab Director, compliance officers, QMS, Quality Assurance and Safety Officer for the Lab. Below is the CLIA / CAP Regulation. CLIA is run by CMS and CAP reports to CLIA. They are the bodies that regulate labs:
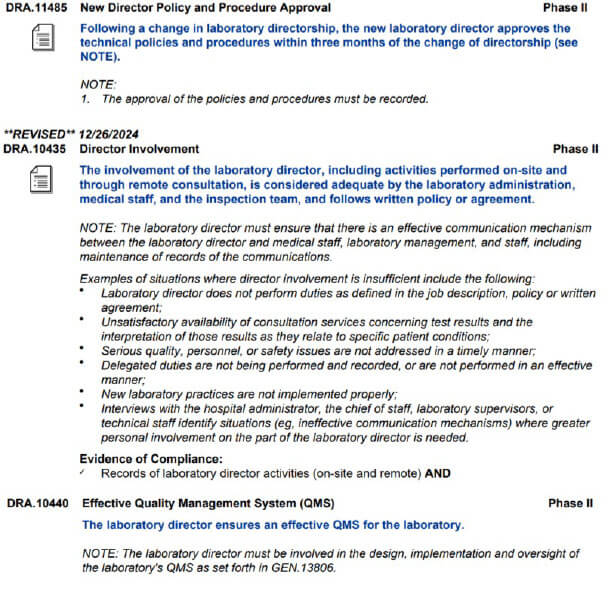
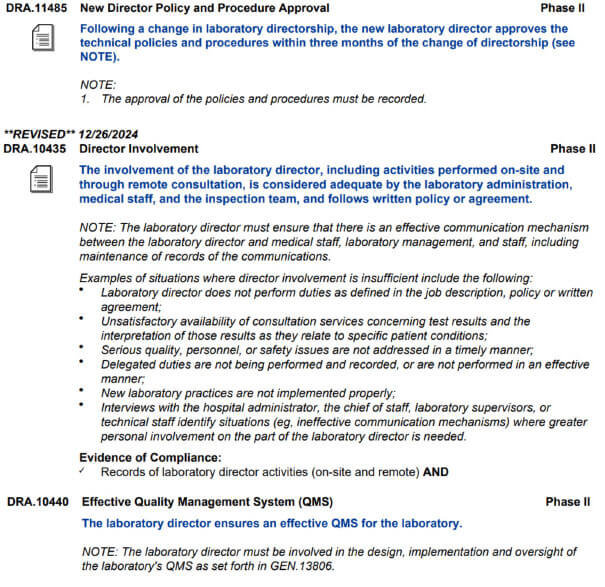
Lab Director Responsibilites
In a CLIA LAB, The Lab Director is Responsible for the entire lab including all regulatory. Elizabeth Holmes is NOT in charge of the Lab regulation neither does the CEO of Quest or LabCorp. The Lab Director, is like the CEO of the actual clinical lab. The buck stops with him. Here’s the Regulation:

Here is a signed official letter form Dr. Adam Rosendorff. He is the same Lab Director at Theranos that caused their lab operation to be put in Immediate patient Jeopardy. Below this letter, is a link to a CBS news story about the mess that went on at that and how the $25M lab facility with their $1.7B state contract was shut down because of Poor Lab Ops by its Lab Director, Dr. Adam Rosendorff, MD. He obviously is not good at Directing a lab.

